Soil Science 101: The Sulfur Cycle

As we all know, sulfur is a macronutrient. Without it, essential plant functions, including photosynthesis, protein and enzyme synthesis cannot occur.
Some agricultural soils have ample sulfur. Others are deficient and require the addition of sulfur fertilizer to ensure healthy, productive crops. So how does sulfur make its way into the soil where it can be utilized by plants? We’ll walk you through the sulfur cycle to explain.
To help us, we draw extensively on the work of John Havlin, Samuel Tisdale, Werner Nelson and James Beaton and their valuable textbook: Soil Fertility and Fertilizers.
Naturally occurring sulfur sources
It’s estimated that the sulfur content in the earth’s crust averages approximately 0.05%, similar to phosphorus.*
Sulfur exists in the soil in organic and inorganic forms. Around 90% of this is organic (in noncalcareous surface soil).*
Note: By ‘organic’ we are referring to sulfur that exists naturally in the soil (ie. not applied as an agricultural fertilizer). This is not to be confused with an organic-certified sulfur fertilizer.
Manufactured sulfur sources
With industrialization, humans were becoming a leading source of sulfur emissions. The production, refining and burning of hydrocarbons (such as coal, crude oil, natural gas and oil sands) all release sulfur dioxide (H2S) into the air.
This atmospheric source of sulfur greatly benefited agriculture production. As the sulfur returned to earth through precipitation, it was a “free” and widely available source of sulfur.
That soon changed. In the 1980s, scientists sounded the alarm. They realized that H2S was causing a significantly detrimental impact on the atmosphere and the planet. Significant steps were taken to reduce industrial emissions through regulations.
Years later, with the reduction of atmospheric sulfur, we are seeing deficiencies in cropland where this macronutrient was once abundant. This has increased the need for sulfur fertilizers.
In a happy twist, the very process developed to remove H2S from oil and gas production and refining (the Claus Process) produces inert organic sulfur as a by-product. Today, this is the primary source of nearly all manufactured sulfur fertilizers.
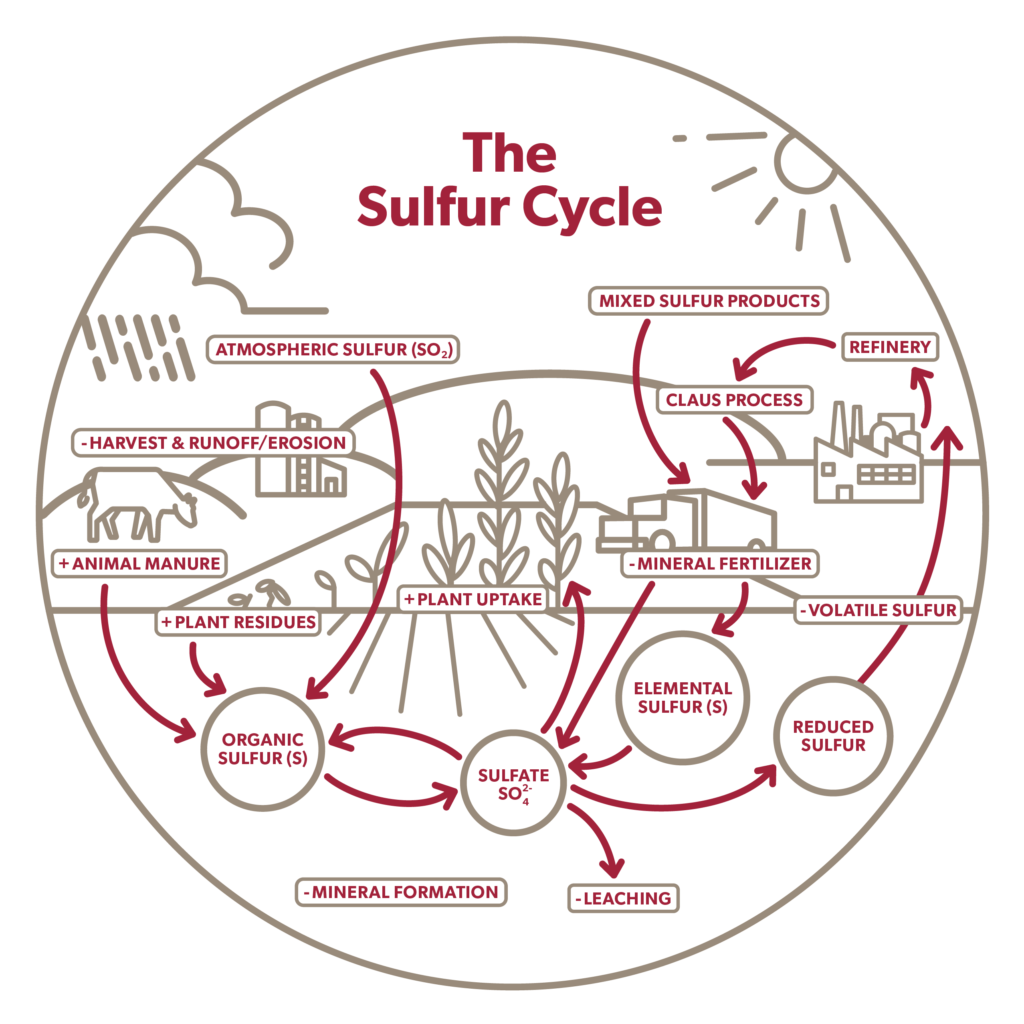
The Sulfur Cycle: This diagram shows how sulfur enters the soil (+) and how it becomes plant available. It also illustrates ways that sulfur sources are lost (-).**
The sulfur cycle: Plant-available sulfur
As we’ve seen, sulfur exists in many forms. Plants cannot utilize elemental sulfur. They require soluble sulfate (SO42-), which is absorbed through their roots. Sulfates are considered an inorganic form of sulfur. There are readily soluble sulfates and adsorbed sulfates (which are released more slowly and tend to be found in the subsoil).
Adsorbed Sulfate: “(Sulfate) reversibly adsorbed by soils and not extracted by water but replaceable by strongly adsorbed anions such as hydroxyl or phosphate.”
P. L. Searle, “Measurement of adsorbed sulphate in soils – effects of varying soils: extractant ratios and methods of measurement.” N.Z. Journal of Agricultural Research 22 (1979): p. 287.
It is important to note that sulfate-S is prone to loss due to leaching and volatilization.
Thiosulfates can also be absorbed through the roots.* Plants can also uptake extremely small quantities of SO2 through their leaves.
As Tisdale, Nelson and Beaton put it in the 4th edition of Soil Fertility and Fertilizers:
“Since plants obtain sulfur primarily from soil as dissolved sulfate, easily soluble sulfate plus adsorbed sulfate represents the readily available fraction of soil sulfur which is utilized by plants.”
Samuel L. Tisdale, Werner L. Nelson and James Beaton. Soil Fertility and Fertilizers (4th Edition): Pearson. 1985.
Most crops require soluble sulfate concentrations of around 3 to 5 ppm in the soil solution. Crops with a high sulfur demand (such as canola, alfalfa and broccoli) will require higher concentrations.*
Microbes convert organic sulfur to sulfate
Most of the sulfur naturally found in soils (over 90%) is in organic forms. Does that mean it can never be used by plants? The answer, of course, is no.
Organic sulfur can be slowly converted into sulfate with the help of microorganisms in the soil. These symbiotic bacteria feed on organic sulfur. Organic elemental sulfur is oxidized by these microorganisms. This produces a sulfate solution.
Once organic sulfur is converted to soluble sulfate, it is plant available. Fortunately, the organic sulfur in soils is slowly converted. This helps to provide a constant supply of sulfur throughout the growing season.
In areas with colder winter climates where year-round cropping does not occur (Canada and the Northern United States), the microbes responsible for sulfur oxidization go dormant in the winter. While the winter weather will break down the sulfur particles into an optimal size required by microbes, the implication is that elemental sulfur fertilizer is not being oxidized and is not subject to leaching losses.
What is considered sulfur-deficient soil?
In North America, at any given time, only between 1-10% of the soil’s sulfur content is easily soluble sulfate.*
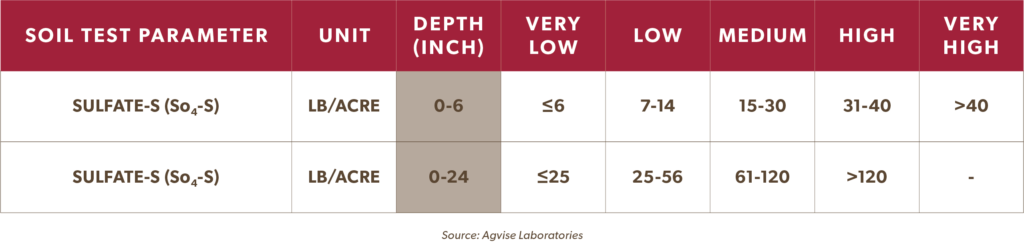
North Dakota-based Agvise Laboratories uses the following soil testing guidelines*** to interpret available sulfur (ie. sulfate sulfur) in the soil. Levels at or below 7-14 lb/acre (at a 6” sampling depth) and at or below 25-56 lb/acre (at a depth up to 24”) are considered sulfur deficient.
Learn more. Watch: Why are Sandy Soils prone to Sulfur Deficiency?
Using sulfur fertilizers in deficient soil.
Sulfur fertilizers are required in sulfur-deficient soils to maintain a high-quality, high-yielding crop. They are also widely used in crops with a high-sulfur demand, such as oilseeds. Another common application is to lower pH in alkaline soils.
Sulfate fertilizers provide an immediately plant-available sulfur source. This includes ammonium sulfate (AMS or AS), single superphosphate (SSP), potassium sulfate, and potassium and magnesium sulfate, as well as gypsum (calcium sulfate). Another option includes liquid sulfur fertilizers such as ammonium thiosulfate, potassium thiosulfate or calcium thiosulfate.
In cases of sulfur deficiency, crops with high sulfur demand, or pH amendment, the most economical sulfur fertilizers are ammonium sulfate and elemental sulfur-bentonite fertilizers.
Farmers will often use a sulfate and elemental product in tandem: applying a small amount of AMS for the early crop stages and relying on microbes to convert elemental sulfur, which becomes available throughout the crop season.
Those who regularly apply elemental sulfur as part of an annual fertility plan can reduce reliance on AMS or eventually replace it altogether.
Keep an eye on your sulfur situation.
While regulations to reduce industrial emissions were introduced decades ago, the impacts are now widespread. Areas of North America where sulfur deficiency was never a concern are seeing the effects today.
The key to managing any nutrient is to remain vigilant. Always be on the lookout for signs of sulfur deficiency: including yellowing leaves (top leaves where new growth is occurring). Make regular crop scouting part of your nutrient cycle!
SOURCES:
* John Havlin, Samuel L. Tisdale, Werner L. Nelson and James Beaton. Soil Fertility and Fertilizers (8th Edition e-book): Pearson. 2014. Chapter 7.
Samuel L. Tisdale, Werner L. Nelson and James Beaton. Soil Fertility and Fertilizers (4th Edition): Pearson. 1985.
** Adapted from: https://set.adelaide.edu.au/fertiliser/ua/media/72/factsheet-sulfur-in-soils.pdf and https://nutrien-ekonomics.com/latest-fertilizer-research/sulfur-transformation/
***Agvise Laboratories: General interpretation of soil test levels.
† P. L. Searle, “Measurement of adsorbed sulphate in soils – effects of varying soils: extractant ratios and methods of measurement.” N.Z. Journal of Agricultural Research 22 (1979): p. 287.




